You have no items in your shopping cart.
Post Requirement
Corrosion is a phenomenon that occurs in metals as a result of a chemical reaction caused by the surroundings leading to the disintegration of the top layer of a metal surface. There are several types of corrosion which take up different forms and mainly depends upon the occurrences that have led to its eventual degradation. Most forms of corrosion can be prevented or slowed down if detected at an early stage. There are several different techniques for the prevention of corrosion. They are:
Regular monitoring of the surrounding environment

Metals are usually not resistant to corrosion in any environment, but careful observation of environmental patterns and the exposure of the metal’s surface can lead to an appreciable decrease in the rate of corrosion.
New alloys are being developed based on previously accumulated data that determines the bearing nature of metals against corrosion. These metals are combined with corrosion-resistant materials that protect the alloy from susceptibility to corrosive environments more than regular carbon steel, etc. Examples of such materials are cobalt, nickel, molybdenum, stainless steel, etc. Structures should be designed so that metals don’t come in contact with reactive materials. Appropriate measures to ensure that no surface is left undefended against corrosion should be taken. Metal surfaces should also be checked to see if there are any scratches, cracks or crevices which increase the rate of eroding.
Inhibitors

Inhibitors are reactants that dissolve into the surface of the metal which acts as a barrier against reactive elements in the environment. Inhibitors work by suspending the chemical process causing corrosion thereby protecting the metal’s surface. Inhibitors are used in the chemical, petroleum and water treatment industries where materials are exposed to corrosion up to a greater extent. Factors that influence the speeding of the inhibitor’s action are:
- Cathodic or anodic nature of the metal
- Reducing the ions scattering on metal’s surface
- Increase in the electrical resistance of the metal
Reducing oxygen, sulfur and chloride content in the surrounding environment

Modifying the surrounding environment is another way of reducing a metal’s susceptibility to corrosion because this process occurs due to chemical reactions of the surroundings with the surface of the metal. A simple way to do this would be to avoid contact with seawater or dampness from any source.
Water fed to boilers in water treatment industries can be treated with chemicals that prevent corrosion or reduce the sulfur and chloride content to avoid corrosion of the inner side of the walls.
Coating

In recent times, paints and coating are being manufactured such that they are corrosion resistant when they come in contact with the environment. Different types of coatings are segregated based on the type of polymer used. Few coatings are
- Water-soluble coatings
- Powder coatings
- High-solid coatings
- Epoxy and alkyd coatings
- Acrylic coatings, etc.
Cathodic Protection
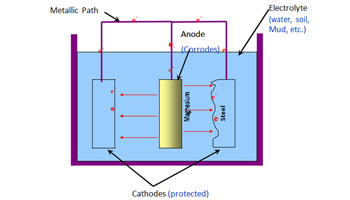
Galvanic corrosion is a type of corrosion that occurs when two different metals are immersed in a corrosive electrolyte. This is a common circumstance when two separate metals are immersed at proximity in damp soil, seawater and other electrolytes. Galvanic corrosion can be seen on ship hulls, near oil rigs, anchors, etc.
Cathodic protection is a form of corrosion resistance where one of the metals act as the anode or active site and the other one acts as the cathode or the passive site. In this process, the surface of the metal to be protected(cathode) is placed in an electrolytic solution with an opposing current with a sacrificial metal(anode). The anodic metal undergoes corrosion while the cathodic metal remains protected. The opposing current pushes the anodes towards polarisation while supplying free electrons to the cathodes.
Plating protection
Plating is a method where the surface of the metal is covered by a plate that hinders corrosion. Plating can also be used for decorative purposes. Some of the common types of platings are:
1.Electroplating

In this type of plating, an electric current is used to transfer the electrons from an electrically charged metal surface on to the underlayer or substrate by forming a thin and lucid coating on the electrode. This is typically done with an anode in its ionic form and a cathode whose electrons are disbursed to form the layer. Both materials are immersed in an electrolytic solution.
2.Electroless Plating
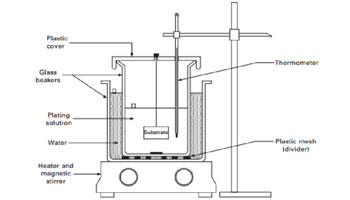
In this non-electric plating method, materials such as nickel, silver, gold, etc.are used as the plating substrate when dipped in a chemical solution.
3.Mechanical Plating

Mechanical plating is a process that transmits the non-corrosive coating by cold welding metal pieces to the piece to be machined. Zinc, aluminium, copper, tin, etc are often used in this process in the form of metal powder added to a metal dipped in a water solution with additives.
Please check out:
Srujana T











sheera
posted on Jan 7, 2020 5:36:04 PM